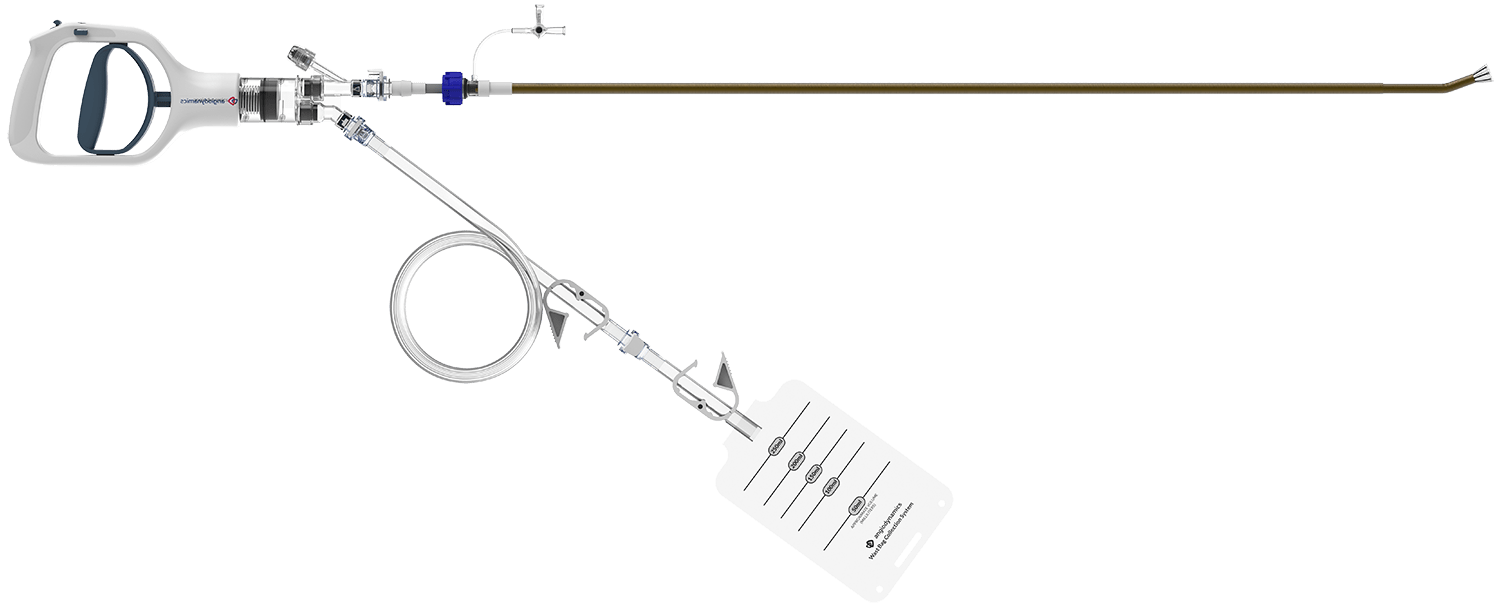
Now Cleared for the Treatment of Pulmonary Embolism
The AlphaVac F1885 System provides procedural time efficiency while maintaining safety & clinical efficacy
For more information on the Pulmonary Embolism indication and the AlphaVac F1885 system, please fill out your information below and your local representative will be in touch with you.
APEX-AV Study Data
25
Sites
122
Patients
35.5%
Mean Reduction in
the Clot Burden†
AlphaVac F1885 US Indication and Risk Information:
Indication for Use:
The F1885 Cannula is indicated for:
- the non-surgical removal of thrombi or emboli from the vasculature
- aspiration of contrast media and other fluids from the vasculature
The Cannula is intended for use in the venous system and for the treatment of pulmonary embolism.
The Handle is indicated as a vacuum source for the AlphaVac Multipurpose Mechanical Aspiration System.
Contraindications:
The following contraindications are applicable:
- The device is contraindicated in the removal of chronic firmly adherent intravascular material.
- The device is contraindicated for use in the right heart or pulmonary arteries during active cardiopulmonary resuscitation.
- The device is contraindicated for blood storage and infusion back into the patient.
Refer to Instructions for Use and/or User Manual provided with the product for complete Instructions, Warnings, Precautions, Potential Complications and Contraindications prior to use of the product.
AngioDynamics, the AngioDynamics logo, AlphaVac, the AlphaVac logo are trademarks and/or registered trademarks of AngioDynamics, Inc., an affiliate or a subsidiary. © 2024 AngioDynamics, Inc.
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. US/VI/WP/2461 Rev.01 04/2024